einococcus bacteria are the most radioresistant organisms known in the world, capable of withstanding doses of gamma radiation a thousand times higher than those fatal to humans. These non-pathogenic bacteria live in all environments, from the glacial granite soils of the Antarctic to burning deserts. This ultra-resistance is the result of their unparalleled DNA repair capabilities, since these bacteria restore their genome broken into a thousand pieces by radiation in only a few hours. Deinococci are also resistant to intense environmental stresses such as ultraviolet radiation and desiccation, an almost total loss of water from the cells.
This curiosity of nature could be useful to medicine after deciphering all these resistance mechanisms, as the work of Arjan de Groot and Laurence Blanchard, respectively CEA and CNRS researchers specializing in genetics and biochemistry, within the Institute of Biosciences and Biotechnologies of Aix Marseille (BIAM), is trying to do. “We have characterized a pair of proteins that play the role of conductor of the response to radiation and desiccation. This is the metallopeptidase IrrE1 and its substrate, the repressor DdrO2, which IrrE cleaves and inactivates after stress inducing the expression of a pool of DNA repair genes,” they say. The missing link was the signal received by the peptidase after stress allowing it to start the repair process without delay. By combining several approaches, the team has just discovered that this signal is in fact a metal, zinc. It shows for the first time in prokaryotes, a secondary messenger role for this metal, crucial after stress.
Zinc at the heart of the virulence of certain pathogens?
In parallel with the discovery of this metallic signal, a literature review conducted by the researchers has brought together a set of concordant results indicating that zinc, a secondary messenger in redox signaling, does not seem to be confined to Deinococcus alone. It may be more universal than currently thought, particularly in the pathogens Listeria and Salmonella. After contact with their natural predators, the macrophages, which induce oxidative stress to defend the organism, a release of zinc in the heart of these pathogenic bacteria seems to promote their virulence, via homologs of IrrE and DdrO.
By understanding how the IrrE and DdrO regulatory protein pair in Deinococcus functions, the researchers are paving the way for the characterization of a new and large family of regulators present in many human pathogens, which could eventually allow us to target their action and control their virulence.
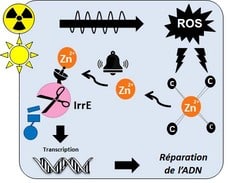
Zinc, a metal that signals stress: In cells, zinc is bound to proteins in which it may have a structural or catalytic role. It is therefore almost never found in a free state within cells. After radiation or desiccation, beyond the DNA, the proteins that bind zinc via cysteines will be damaged by the reactive oxygen species (ROS) generated by these stresses. Zinc, in the form of the divalent cation Zn2+, is then released and becomes accessible to other, less affine proteins, including IrrE, which becomes active and immediately initiates the cascade of events that leads to the repair of damaged DNA and the survival of bacteria (Figure 1)
REFERENCES
Redox signaling through zinc activates the radiation response in Deinococcus bacteria
Romaric Magerand, Pascal Rey, Laurence Blanchard, Arjan de Groot* Sci Rep. 2021 Feb 25;11(1):4528. doi: 10.1038/s41598-021-84026-x.
1Ludanyi, M.1, Blanchard, L.1, Dulermo, R., Brandelet, G., Bellanger, L., Pignol, D., Lemaire, D., & De Groot, A.* (2014). Radiation response in Deinococcus deserti: IrrE is a metalloprotease that cleaves repressor protein DdrO. Mol. Microbiol. 94(2):434-49. DOI: 10.1111/mmi.12774
2.De Groot, A., Siponen, M.I., Magerand, R., Eugenie, N., Martin-Arevalillo R., Doloy, J., Lemaire, D., Brandelet, G., Parcy, F., Dumas, R., Roche, P. Servant P., Confalonieri, F., Arnoux, P., Pignol, D., Blanchard, L.,* (2019) Crystal structure of the transcriptional repressor DdrO: insight into the metallo